by H. Chung So, Public Information Officer
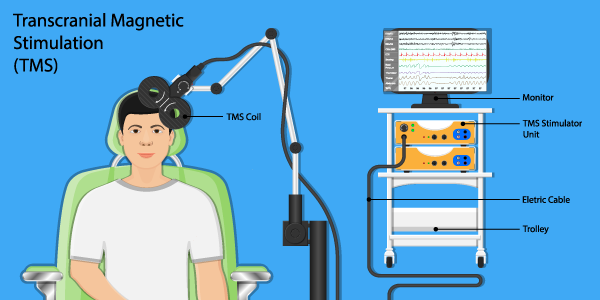
Thanks to support from California’s Mental Health Services Act (MHSA), LACDMH has been able to provide transcranial magnetic stimulation (TMS) to treat clients experiencing depression symptoms that are resistant to first-line treatment, such as medication and psychotherapy. While TMS was approved by the Food and Drug Administration to treat depression in 2008, it has only been primarily available in private healthcare systems, and LACDMH is one of the first public mental health systems to offer this safe and highly effective treatment for our clients.
With TMS, a magnetic coil is placed on the surface of the client’s head and it is then activated to generate a magnetic field to stimulate specific areas of the brain, providing immediate relief from symptoms as well as long-term relief with repeated applications (a typical course of treatment is five TMS sessions a week over four to six weeks.)
“TMS is very effective for treating depression, and is being studied at other organizations for other behavioral conditions such as bipolar disorder, schizophrenia, post-traumatic stress disorder, substance use disorders, and suicidal crises,” said LACDMH Associate Medical Director Marc Heiser, M.D., Ph.D. “We are excited to be able to offer TMS for clients who have not responded as well to first-line interventions for depression.”
Heiser also emphasized that TMS is not electroconvulsive therapy (ECT) – which involves directly applying electricity to the brain to induce a brief, controlled seizure while the client is asleep under anesthesia. Unlike ECT, clients remain awake during TMS sessions, and it is generally better tolerated with fewer and less severe side effects compared to ECT.
“The magnetic pulses are delivered in very short bursts, and it feels like a tapping on the head,” Heiser said. Most common TMS side effects include headaches and lightheadedness, and they are typically mild and brief. In contrast, side effects of ECT are more intense and can include nausea, muscle aches, and memory loss.
As part of MHSA’s Innovation Project series—which introduces and implements novel mental health treatments and practices to improve service delivery and clinical outcomes—LACDMH has been able to acquire and operate one TMS machine and has treated approximately 60 clients. Based on the positive outcomes of this project, with most patients experiencing sustained relief from symptoms with minimal side effects, LACDMH hopes to acquire more TMS machines and staff so more clients in L.A. County can access this safe and highly effective treatment.
Clients surveyed about their level of pain/discomfort on a 0-10 scale reported an average of “2” during the TMS session and “1” after the session. Most clients also reported being satisfied or very satisfied with TMS treatments, and clinical evaluations of depression symptoms have shown improvement following TMS treatment with a significant reduction in symptom severity.
Given the proven benefits of TMS in the current MHSA project – Heiser said LACDMH plans to apply for additional MHSA innovation project support to acquire more TMS machines and hire staff to operate them. This, as well as Medicaid’s upcoming coverage of TMS treatments in July 2023, would expand its availability to more clients throughout L.A. County. Furthermore, Heiser said FDA may approve TMS to treat additional behavioral conditions given its promising results from clinical trials, expanding its use potential for a wider group of clients. (Note: LACDMH is only providing TMS therapy for FDA-approved uses and is not a participant in these clinical studies.)
Currently, clients of LACDMH directly operated clinics can speak to their mental health care provider to be considered for TMS therapy for depression. Once additional machines and staff are available and online, Heiser expects our department to be able to provide TMS therapy to clients of LACDMH contract providers as well. To learn more about this treatment, visit the TMS page on the National Institute of Mental Health’s website.




